Allodynic Therapeutics, Inc. is a clinical-stage pharmaceutical company focused on developing naltrexone-acetaminophen combination for treating acute migraine and co-occurring anxiety. The company has completed four investigator-initiated clinical trials that have provided promising results in treating various pain conditions and is now launching a phase-2 clinical study for treating acute migraine. The naltrexone-acetaminophen combination US patents extend to 2037
About Allodynic Therapeutics, Inc.
Benefits of the Naltrexone-Acetaminophen Combination in Combating the Opioid Crisis

Key Points:
-
- Potential effectiveness in treating neuropathic pain, which includes conditions like back pain, migraine, postherpetic neuralgia, and trigeminal neuralgia.
- Neuropathic pain is a challenging type of pain to manage, affecting 24 million Americans, while 38 million suffer from migraines.
- Current common treatments involve the prescription of opioids, which have limited effectiveness and a substantial risk of developing addiction.
- Despite their limited effectiveness, a significant percentage of neuropathic pain patients (76%) are prescribed opioids.
- Implementing the naltrexone-acetaminophen combination could prevent 10% of premature deaths from opioid overdose, potentially saving 24,700 lives over a 20-year period.
Key Attributes of the Naltrexone-Acetaminophen Combination
Main Points:
- Research indicates that both naltrexone and acetaminophen individually are effective in treating acute migraines, physical pain, and emotional pain (References 1-15).
- In a clinical study, the naltrexone-acetaminophen combination demonstrated greater emotional pain relief compared to the sum of the individual components, suggesting a synergistic effect in the treatment of emotional pain.
Overview of Four Phase-2, Randomized, Placebo-Controlled Clinical Studies for the Naltrexone-Acetaminophen Combination
Study 1: Acute Migraine factorial study involving 72 patients across five treatment groups. (Link to preprint)
Study 2: Acute Migraine study with 36 patients in three treatment groups.
Study 3: Nine-month Migraine Prevention study, included twelve patients in two treatment groups, with an extension phase for non-responders. (Link to preprint)
Study 4: Nine-month Chronic Low Back Pain study, involved twelve patients in two treatment groups, with an extension phase for non-responders.
Highlights from Clinical Studies of the Naltrexone-Acetaminophen Combination
Key Findings:
-
- Acute Migraine Factorial Study: Naltrexone 2.25 mg (n=19) demonstrated a 17.3% higher response rate for pain freedom at 2 hours compared to placebo (n=17) (Reference 14).
- Migraine Prevention Study: 67% of patients receiving twice daily naltrexone 2.25 mg-acetaminophen 325 mg (N=6) experienced a 75% reduction in migraine days, compared to 17% of placebo patients (N=6) (Reference 15).
- Nine-month Migraine Prevention and Chronic Low Back Pain Studies: ALLOD-2 showed a 50% higher response rate than placebo in achieving a ≥50% reduction in the Pittsburgh Insomnia Rating Scale-20 (References 14-15).
Milestones for the Naltrexone-Acetaminophen Combination Development
 Key Achievements:
Key Achievements:
- Investigator-initiated acute migraine studies: two studies involving 108 patients.
- Migraine prevention study: nine-month duration.
- Chronic low-back pain study: nine-month duration.
- FDA Type B, face-to-face meeting held on July 21, 2019.
- Initial Pediatric Study Plan accepted by the FDA.
- FDA breakthrough designation for naltrexone-clonidine combination in 2013.
- Two orphan drug designations.
- Submission of five Clinical Study Reports to the FDA.
Intellectual Properties and Market Advantages of the Naltrexone-Acetaminophen Combination
Key Points:
-
- The naltrexone-acetaminophen combination is protected by three U.S. patents (9,095,548, 9,707,225, and 11,058,68).
- The naltrexone-ibuprofen combination holds one patent (9,205,081).
- Market advantages include established public trust in acetaminophen and the use of “low dose” naltrexone.
- A potential for over-the-counter “Super Tylenol.”
Mechanism of Action of the Naltrexone-Acetaminophen Combination
Key Points: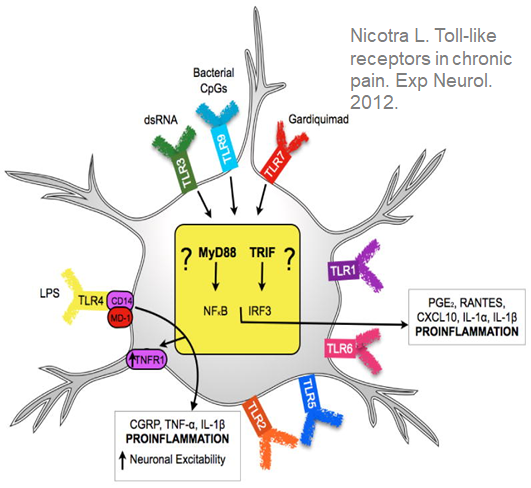
- Naltrexone (NTX) is thought to reduce pain by blocking the Toll-Like Receptor 4 (TLR4), which lowers pro-inflammatory cytokine levels associated with pain.
- TLR4 is found in the Dorsal Root Ganglia (DRG) and trigeminal ganglion neurons.
- Activation of TLR4 leads to pro-inflammatory cytokines (e.g., CGRP, TNF-Alpha, IL-1 Beta, NO, ROS) that cause neuropathic pain.
- Naltrexone suppresses cytokine production, acting at the origin of the TLR4-activated cascade and preventing the production of multiple cytokines.
- Individual cytokine blockers (CGRP and TNF-alpha inhibitors) act downstream from naltrexone, blocking only one of many neuropathic pain-generating cytokines.
- Acetaminophen is believed to work by inhibiting cyclooxygenase-2 (COX-2), leading to reduced PGE2.
- The naltrexone-acetaminophen combination targets multiple biological pathways, simultaneously reducing the production of pro-inflammatory cytokines and PGE-2.
Introduction to Allodynic's Founder

Welcome. I’m Dr. Annette Toledano, a board-certified internist with over three decades of experience in patient care. My journey with Low-Dose-Naltrexone (LDN) began when it brought considerable relief to my personal battle with back pain. This encounter with LDN inspired the inception of Allodynic Therapeutics.
Thirteen years ago, I embarked on developing a medication designed to block pain-inducing chemicals called cytokines. While the endeavor was a departure from the status quo, my objective remained clear: to envision and facilitate a future with diminished pain, focusing on individual patient needs.
I hold a medical degree from the Faculty of Medicine at Technion – Israel Institute of Technology in Haifa, Israel. My dedication to naltrexone-acetaminophen is steadfast as I continue my efforts to bring meaningful change to pain management.

References
- Hutchinson MR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone. Eur J Neurosci. 2008.
- Nicotra L. Toll-like receptors in chronic pain. Exp Neurol. 2012.
- Ellis A. Systemic Administration of Propentofylline, Ibudilast, and (+)-Naltrexone Each Reverses Mechanical Allodynia in a Novel Rat Model of Central Neuropathic Pain. J Pain. 2014.
- Lewis SS. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J Pain. 2012
- Wang X. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol. 2016.
- Wieseler J. Supradural inflammatory soup in awake and freely moving rats induces facial allodynia that is blocked by putative immune modulators. Brain Res. 2017
- Selfridge BR. Structure-Activity Relationships of (+)-Naltrexone-Inspired TLR4 Antagonists. J Med Chem. 2015
- Marmura MJ. The acute treatment of migraine in adults: the american headache society evidence assessment of migraine pharmacotherapies. Headache. 2015
- Lipton RB. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000
- Roberts ID. Acetaminophen influences social and economic trust. Sci Rep. 2019
- Dewall CN. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010
- Wardle MC. Naltrexone alters the processing of social and emotional stimuli in healthy adults. Soc Neurosci. 2016
- Devlen J. Anxiety and depression in migraine. J R Soc Med. 1994
- Toledano AC. The Acute Treatment of Migraine with Low-Dose Naltrexone and Acetaminophen Combinations and Each Component: Findings of a Small, Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. MedRxiv. 2021
- Toledano AC. The Preventive Treatment of Migraine with Low-Dose Naltrexone and Acetaminophen Combination: Findings of a Small, Randomized, Double-Blind, and Placebo-Controlled Clinical Trial with an Open-Label Extension for None-Responders. MedRxiv. 2021
Published Preprints of our Clinical Trials
The Acute Treatment of Migraine with Low-Dose Naltrexone and acetaminophen Combinations and Each Component: Findings of a Small, Randomized, Double-Blind, and Placebo-Controlled Clinical Trial – https://medrxiv.org/cgi/content/short/2021.03.22.21254145v3
The Preventive Treatment of Migraine with Low-Dose naltrexone and acetaminophen Combination: Findings of a Small, Randomized, Double-Blind, and Placebo-Controlled Clinical Trial with an Open-Label Extension for None-Responders – https://medrxiv.org/cgi/content/short/2021.03.23.21254186v3
Our Studies on Clinicaltrials.gov
- A Phase 2 Study Evaluating Naltrexone-Acetaminophen in Acute Migraine and Co-Occurring Anxiety in Adults (Vivifiq)
- Low-Dose Naltrexone and Acetaminophen Combination and Its Components in the Acute Treatment of Migraine (ANODYNE-1)
- Low-Dose Naltrexone and Acetaminophen Combination and Sumatriptan in the Acute Treatment of Migraine With Nausea (ANODYNE-2)
- The Preventive Treatment of Migraine With Low-Dose Naltrexone and Acetaminophen Combination (ANODYNE-3)

- Low-Dose Naltrexone and Acetaminophen Combination in the Treatment of Chronic Low Back Pain (ANODYNE-4)
- Naltrexone and Clonidine Combination (ATNC05) in the Treatment of Chronic Back Pain


